Tissue Response to Expanded Polytetrafluoroethylene and Silicone Implants in a Rabbit Model
Rami K. Batniji, MD; Joseph L. Hutchison, MD; Ravi Dahiya, MD; Samuel L. Lam; Edwin F. Williams III, MD
Background: Expanded polytetrafluoroethylene (ePTFE) and silicone are safe and relatively biocompatible materials.
Objective: To compare, using multiple histologic parameters, the tissue response to a standard silicone soft tissue implant with the response to a modified ePTFE implant. The modified form of ePTFE is reinforced with fluorinated ethylene propylene (FEPRePTFE), which provides increased pliability and material integrity.
Methods: The implants were placed into a subperiosteal pocket over the skull of adult New Zealand white rabbits. At 7, 30, and 90 days after implantation, en bloc tissue specimens, including skin, implants, surrounding soft tissue, and underlying bone were harvested for gross and histologic evaluation.
Outcome Measures: The tissue response to the implants was assessed with respect to the number of foreign body giant cells present, the thickness of the fibrous capsule, and the general inflammatory response (n=6 for each implant at each evaluation period).
Results: There were no cases of rejection, extrusion, or infection. The silicone implants elicited a significantly thicker capsule and less neovascularization (P<0.5)
Conclusion: The FEPRePTFE demonstrated a favorable tissue response when compared with silicone, particularly in regard to capsule thickness and vascular ingrowth.
Arch Facial Plast Surg. 2002; 4: 111-113
VARIOUS ALLOPLASTIC materials are currently used for aesthetic and reconstructive soft tissue augmentation of the face. Among these alloplastic implants are silicone and expanded polytetrafluoroethylene (EpTFE). Initially used for vascular grafts and subsequently for hernia repair, ePTFE has now been used relatively safely for more than 20 years. 1,2 More recently, use of ePTFE has been well documented for facial bony augmentation. 3-5 Several clinical studies have demonstrated the potential advantages of EpTFE. The 2 most significant advantages are rapid stabilization and limited capsule formation. 6-8 The purpose of our study was to examine the histologic response to a new formulation of EpTFE, one reinforced with fluorinated ethylene propylene (FEPRePTFE). This change in configuration has demonstrated improved carvability and firmness and could prove to be advantageous to clinical application. 9 However, adverse effects of any new material must be assessed prior to widespread clinical use.
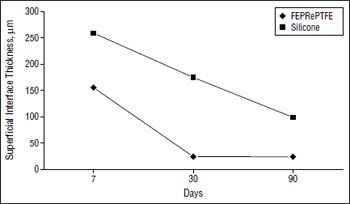
Figure 1. At superficial interface, while there is a trend toward a thinner capsule with both materials, at all evaluation points the silicone elicits a significantly thicker capsule than the expanded polytetrafluoroethylene reinforced woth fluorinated ethylene propylene (FEPRePTEFE).
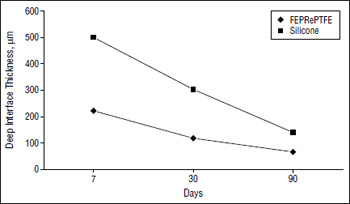
Figure 2. At deep interfce, there is a more vigorous capsule formation around the silicone implant than around the implant of expanded polytetrafluoroethylene reinforced with fluorinated ethylene propylene (FEPRePTFE).
The biocompatibility of FEPRePTFE has not yet been well described. The histologic response of this new material was compared with that of silicone in this study.
RESULTS
None of the implants extruded. There was no case of postoperative wound infection, dehiscence, or hematoma. Also, no statistically significant difference was found between the 2 implants in regard to foreign body giant-cell response or number of macrophages at any evaluation period (P=.44,.17, and .58 for foreign body giant-cell response at 7, 30 and 90 days, respectively; P=.19, .15, and .22 for number of macrophages at 7, 30 and 90 days, respectively). Neither material resulted in any significant bone resorption during the study period. The number of peri-implant blood vessels that formed did not vary between the 2 implants at day 7 and day 30. However, by day 90, significantly more neovascularization had occurred in the FEPRePTFE group (P=.004). The capsule formation, as measured by the deep and superficial interface thickness, was significantly thicker around the silicone implants at each of the evaluation periods (P<.001 for deep interface at 7 days; P=.004 and .003 for deep interface at 30 and 90 days, respectively; P=.01, .002, and .002 for superficial interface at 7,30 and 90 days, respectively). This difference is illustrated in Figure 1 and Figure 2, which also demonstrate that the thickness decreased over time in both cases.
COMMENT
Facial reconstruction with alloplastic materials has been described and performed for centuries. A wide range of materials have been tried including cork, paraffin, ivory, gold, and silver. However, most of these experiments have demonstrated the alloplastic materials to be unsuitable. While autologous materials are often preferred, they can be associated with problems such as absoption, donor site morbidity, warping, increased operative time, and insufficient donor material.
The past century has witnessed the advent of many synthetic polymers, few of which have stood the test of time. Silicone and ePTFE are among the most widely used polymers and are well described in the facial plastic surgery literature. These materials have many of the properties first described by Scales 10 as being ideal for an implant. Among others, these properties include being chemically inert, noncarcinogenic, unmodified by soft tissue, pliable, and firmly resistant to strain. A relatively new form of ePTFE, FEPRePTFE was designed with the purpose of enhancing 2 of those ideal properties, namely, pliability and firmness. Our literature review failed to yield any studies on the histologic response to FEPRePTFE. Given these improved physical attributes, our goal was to determine if the tissue response histologically would reveal any adverse reactions. The initial evaluation was performed at 7 days to examine differences in the acute response. Since normal healing in rabbits is complete in 14 to 16 days, 11 any inflammatory reaction at 30 and 90 days could be assumed to be due to the implant.
As the results indicated, none of the implants were extruded or infected. We also found no evidence of bone resorption during the study period. The most notable and consistent difference between the 2 implants concerned the thickness of the surrounding capsule. By day 90, nearly a 4-fold difference in the superficial interface thickness had developed, with the silicone implant eliciting a thicker capsule.
One other difference that was noted on histologic examination, but not quantified among our measured outcomes, was the more abundant collagen ingrowth in the FEPRePTFE implants than in the silicone implants (Figure 3 and Figure 4). This finding correlates with the higher vascular migration in the FEPRePTFE implants, which was statistically significant by day 90. These characteristics are thought to be the reason behind the more rapid stabilization of this material. 8 Although stabilization is desirable, the ideal implant can also be easily removed if necessary. A limited capsule formation may facilitate this property. We noted a dramatic and statistically significantly thinner capsule around the FEPRePTFE implant.
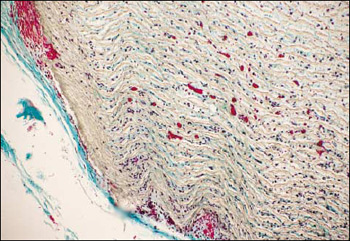
Figure 3. This slide demonstrates a thin, bland, fibrous capsule around the implant of expanded polytetrafluoroethylene reinforced with fluorinated ethylene propylene (FEPRePTFE). Also seen in the interstices are numerous cells, capillaries, and collagen.
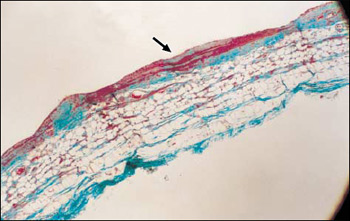
Figure 4. The silicone implant elicits a thick, dense fibroadipose capsule (original magnification x 10).
In conclusion, the study demonstrates that FEPRePTFE, a new configuration of ePTFE, is characterized by a favorable vascular migration and limited capsule formation. These traits of the FEPRePTFE are advantageous in an implant candidate. The silicone elicited a more vigorous capsule formation and less neovascularization. No significant adverse reactions occurred in either implant. However, the relatively short 3-month examination period may not account for longer-term adverse effects such as implant migration, extrusion, or infection. Histologic analysis of ePTFE suggests that these complications are unlikely. Further clinical studies will more clearly delineate the benefits of FEPRePTEFE in facial plastic surgery.
Accepted for publication October 31, 2001
This study was presented at the Combined Otolaryngologic Spring Meeting, Orlando, Fla, May 13, 2000
Corresponding author and reprints: Edwin F. Williams III, MD, Division of Otolaryngology, Albany Medical Center, 47 New Scotland Ave, Albany, NY 12208
REFERENCES
- Soyer T, Lempinen M, Cooper P, Norton L, Eiseman B. A new venous prosthesis. Surgery. 1972;72:864-872
- Jenkins SD, Klamer TW, Parteka JJ, Condon RE. A comparison of prosthetic materials used to repair abdominal wall defects. Surgery. 1983;94:392-398.
- Sherris DA, Larrabee WF. Expanded polytetrafluoroethylene augmentation of the lower face. Laryngoscope. 1996;106:658-663
- Maas CS, Gnepp DR, Bumpous J. Expanded polytetrafluoroethylene (Gore-Tex soft tissue patch) in facial augmentation. Arch Otolaryngol Head Neck Surg. 1993:119:1008-1014.
- Krzystof C, Giman G. A 6-year experience with the use of expanded polytetrafluoroethylene in rhinoplasty. Plast Reconstr Surg. 1998;101:1675-1683.
- Godin MS, Waldman SR, Johnson CM. The use of expanded polytetrafluoroethylene (Gore-Text) in rhinoplasty. Arch Otolaryngol Head Neck Surg. 1995;121:1131-1136.
- Maas CS, Merwin GE, Wilson J, Frey MD, Maves MD. Comparison of biomaterials for facial bone augmentation. Arch Otolaryngol Head Neck Surg. 1990;116:551-556.

